Creating a new medicine takes time. But time is a luxury many patients can’t afford.
With this urgency in mind, Scripps Research has pioneered a new nonprofit research model to dramatically accelerate the translation of groundbreaking scientific discoveries into life-changing therapies. Over the past several years, Scripps Research—which has originated nine approved drugs—has greatly expanded its translational research capacity. Under the leadership of Peter Schultz, PhD, the institute is simultaneously fueling fundamental scientific discoveries that give new insights into human diseases and speeding their translation into new medicines.
At the heart of this evolution is the merger of Scripps Research with two other entities: Calibr, a nonprofit drug discovery center founded by Schultz in 2012; and the Scripps Research Translational Institute founded by Eric Topol, MD, to individualize healthcare by leveraging the remarkable progress being made in human genomics and combining it with the power of wireless digital technologies and artificial intelligence.
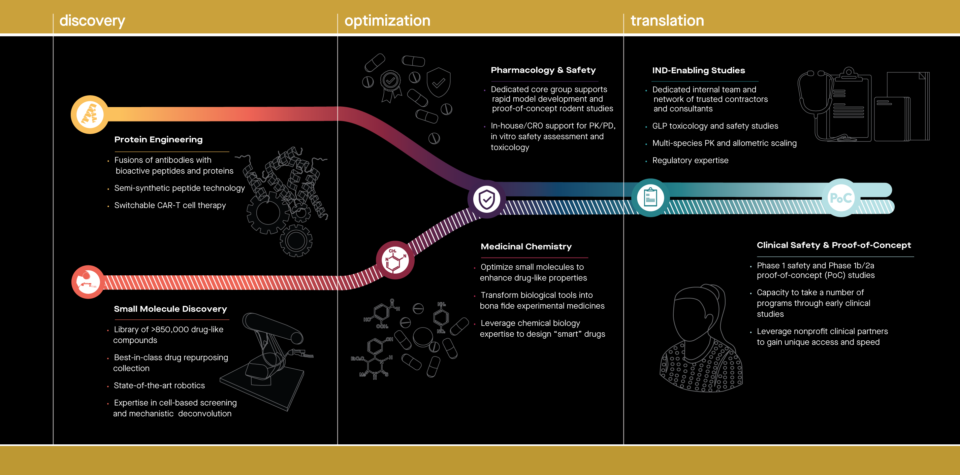
The addition of Calibr, which specializes in the identification, optimization and early-stage clinical testing of drug candidates, provides a seamless path for advancing promising Scripps Research discoveries into human trials. Recently, the institute launched Primer, a novel funding vehicle that will be used to advance a portfolio of candidate medicines to treat cancer, osteoarthritis and neurodegenerative, cardiopulmonary and infectious diseases. Initial funding has been committed through a lead grant from the Bill & Melinda Gates Foundation, and most recently, the Hearst Foundations. The investments in Primer are made as recoverable grants that are returned to contributors upon licensing of programs in the Primer portfolio for further development.
Scripps Research has created this novel approach not only to change the way new medicines are developed, but also to ensure the long-term sustainability and growth of nonprofit research by capturing more of the value in its science and reinvesting it back into research. “Primer enables us to develop innovative medicines that impact public health and creates an evergreen model for funding basic and translational research in the nonprofit sector,” says Schultz, president and CEO of Scripps Research and Calibr.

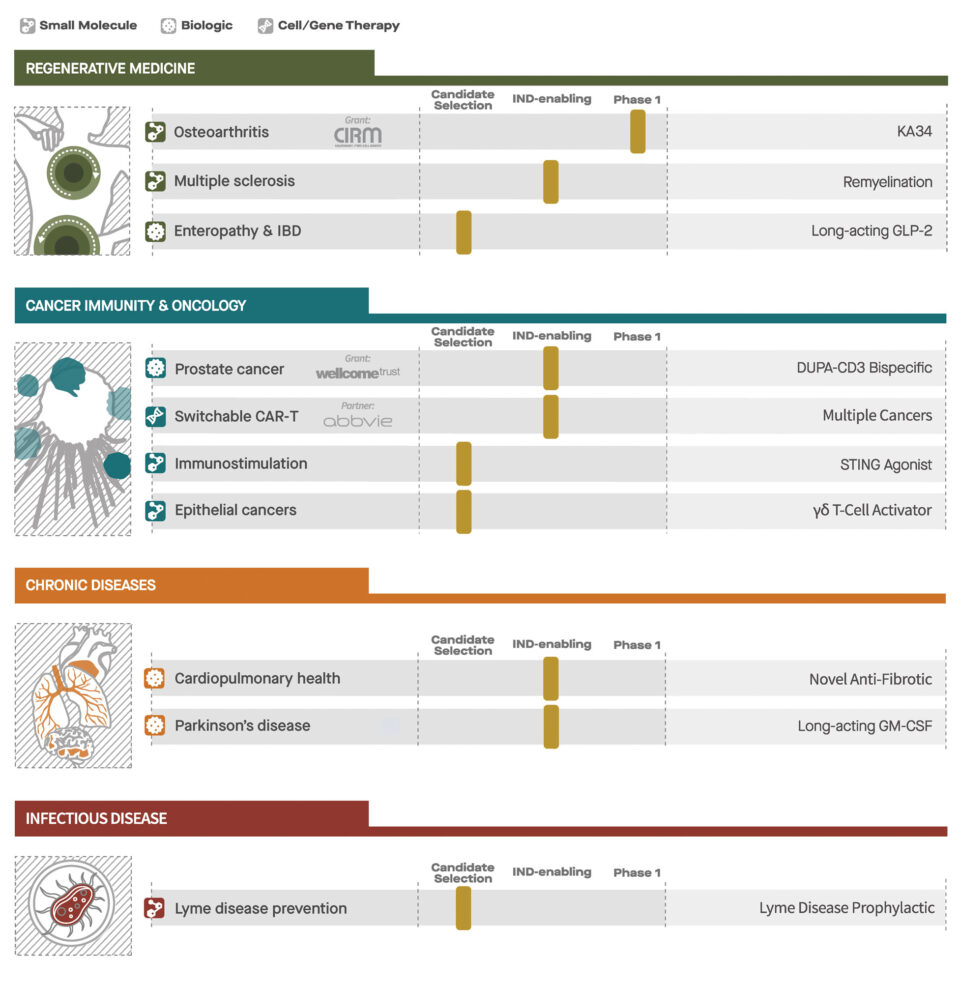
The initial Primer portfolio includes 10 select drug candidates in varying stages of development. Among the assets in the initial Primer-funded portfolio is KA34, a small molecule that stimulates cartilage production and repair in osteoarthritis, the prevalent form of arthritis that affects tens of millions of people worldwide. The only nonsurgical therapies for the disease are drugs that treat the pain and inflammation but have no impact on the loss of cartilage, the underlying cause of the disease.
The idea for KA34 stemmed from a discovery of a compound at Scripps Research that generates chondrocytes, specialized cells that produce healthy cartilage in the knee joint. When given to animals, the compound was effective at regenerating cartilage. KA34 was created as the result of medicinal chemistry optimization of physical properties and toxicology studies in animals.
“The story of KA34 is proof positive that integrating academic science and drug discovery capabilities offers incredible potential for speeding translation of basic discoveries into medicines in the nonprofit sector,” says Schultz. KA34 is being tested as a treatment for osteoarthritis of the knee in a phase 1 clinical trial run by Calibr scientists and funded by the California Institute for Regenerative Medicine (CIRM). Another regenerative program for multiple sclerosis has been partnered to advance into a phase II clinical trial.
Primer will also support the clinical evaluation of DUPA-CD3, a novel bispecific antibody for metastatic prostate cancer that harnesses the patient’s own immune system to eliminate tumor cells. Calibr expects to begin treating patients with DUPA-CD3 in the second half of 2019.
Primer’s other lead cancer program is a “switchable” Chimeric Antigen Receptor T-cell (CAR-T) cancer therapy, which will be advanced by the Calibr team into a phase 1 clinical trial in cancer patients this year in partnership with AbbVie. Calibr’s innovative CAR-T therapy program is designed to enhance safety, versatility and efficacy through a CAR-T cell that uses antibody-based molecules to control the activation and antigen specificity of CAR-T cells. AbbVie entered into a significant partnership with Calibr with the belief that this technology will allow CAR-T therapy to safely treat solid tumors, such as breast and prostate cancer, which have eluded conventional CAR-T therapies to date.
Primer includes two additional immune-based therapies for cancer as well as candidate medicines for degenerative disease and chronic diseases of aging. These latter programs include a novel immunomodulatory therapy for Parkinson’s disease, an engineered peptide that stimulates regeneration of the intestinal barrier for gastrointestinal disease, a drug for lung fibrosis, and a long-acting oral preventative for Lyme disease.
The early stages of drug development—identifying and optimizing lead drug candidates, preclinical studies and early human trials for safety and efficacy—are notorious for presenting significant barriers to progress. These stages are commonly referred to as the “valley of death” by researchers, a reference to the challenges of finding public and private funding and partners with translational research expertise for an as-yet untested experimental medicine. Many promising ideas for new therapies stall due to these barriers.
Scripps Research Professor Hugh Rosen, MD, PhD, says in his experience it required tremendous effort to bring a laboratory discovery to the point of testing a drug in clinical trials. Discoveries by Rosen and another Scripps Research professor, Edward Roberts, PhD, in the early 2000s led to the drug ozanimod, which is expected to be approved by the U.S. Food & Drug Administration for the treatment of multiple sclerosis later this year.
“The translational scientific infrastructure and expertise at Scripps Research are now established and funding mechanisms already in place, which will help overcome many of the hurdles we faced in the past,” Rosen says. “It serves everyone well— the scientists, the institute and, most importantly, patients.”
Primer
New medicines on the horizon
Leveraging the unique scientific framework of Scripps Research, Calibr has created a portfolio of drug candidates based on Scripps technologies, and is shaping a new paradigm for advancing nonprofit biomedical research to impact patients while re-investing in further innovative research. Primer supports taking advanced assets through IND-enabling studies and early clinical development. Aiming to accelerate an impact on patients and create significant value to support scientific research, Primer advances a portfolio of new medicines to treat cancer, degenerative diseases, and chronic diseases that affect either children or the aging population.
Calibr Capabilities and Infrastructure
From bench to bedside