Scientists tackle the mystery of how the brain listens to the body
Neuroscientists rapidly decode interoception, the long-overlooked links between our brain and the heart, stomach and other organs
How do you feel right now? Does your back ache from sitting at a computer too long? Are you hungry? Thirsty? Nervous?
Whether you are conscious of it or not, your brain is constantly checking in with your body to see how things are going and helping you make decisions about the impending future.
This interior dialogue between your body and your nervous system, referred to by scientists as “interoception,” is fundamental to your conscious experience. It’s also essential to more under-the-radar processes that help your heart, lungs, stomach and other organs respond to your minute-to-minute needs without you having to make any conscious effort. Yet, vital as it is, scientists know very little about how interoception works.
“It’s really remarkable how little we know about these internal sensory systems compared to what we know about our external senses,” says Ardem Patapoutian, PhD, a neuroscience professor at Scripps Research and winner of the 2021 Nobel Prize in Physiology or Medicine. “We’re just now beginning to identify how these neurons gather information from our organs, to decipher the neural circuits that process that information before it goes to the brain, and to figure out what the brain does with that information.”
Recently, Patapoutian and other neuroscientists at Scripps Research set out to map the labyrinth of neurons that innervates our internal organs and to decode the flow of information they carry to and from the brain.
The Scripps Research team, armed with cutting-edge research technologies developed over the past decade, is making rapid advances in this long-neglected field of neurobiology. The stakes are high.
Our interoception systems help us stay balanced while we walk, keep our blood pressure and heart rate steady, play a key role in hunger and thirst, and appear to influence our moods and emotions. They are involved in many of the essential biological functions that are undermined by diseases affecting billions of people worldwide.

“We’re just now beginning to identify how these neurons gather information from our organs, to decipher the neural circuits that process that information before it goes to the brain, and to figure out what the brain does with that information.”
-Ardem Patapoutian, PhD
Under Pressure
Patapoutian’s entrée into the field of interoception was via a deceptively simple idea with far-reaching consequences.
A little over a decade ago, his lab was hunting for elusive quarry, the molecules on the surface of neurons that sense touch. At the time, it was known that neurons somehow responded to the mechanical force of, say, a feather brushing against a person’s skin, but the molecular-scale machinery that enabled this response was unknown. Patapoutian’s team posited the central question: What happens to a neuron when you poke it? Determined to find an answer, they used a method of jabbing single cells with a tiny glass probe in a laboratory dish. This pressure forced the cells to fire off an electrical signal—the essential information currency of the nervous system. Then, one by one, using genetic manipulation technologies, the scientists removed different types of proteins, known as ion channels, from the neurons to find the sensor that responds to the applied mechanical force.
Ion channels act as gates in a nerve cell’s outer membrane, letting electrically-charged ions in or out, which changes the neuron’s electrical state depending on whether the gate opens or closes. Through this process of elimination, Patapoutian and his team discovered two ion channels that were necessary for the cells to respond to the jabs from the probe. They’d found what they—and many other scientists—were looking for: the missing link in the neurological chain of events that allows us to feel touch sensations, from a gentle caress on our skin to the wind in our hair. They named the two channels PIEZO1 and PIEZO2.
It turns out the channels were involved in far more than the sense of touch. They have since been found in many internal organs, including the heart, lungs, blood vessels and lining of the stomach, to name a few. Patapoutian’s team even recently found them in the roots of plants — which is a whole other story.
Why would we have pressure sensors in our organs? As it turns out, many physiological functions involve mechanical forces that our brain and other parts of our nervous system must monitor and influence. The pumping of our heart and lungs, for example, requires those tissues to stretch and contract. Patapoutian’s PIEZO receptors help the brain keep track of heart rate and breathing. Blood vessels also shrink or swell depending on our moment-to-moment needs. Every time you stand up, blood vessels throughout your body must, in an instant, simultaneously contract so that enough blood keeps flowing to your now-elevated brain. PIEZOs help with this too. They might also be involved in tracking how much your stomach stretches when you eat and the amount of food passing through your intestines as you digest your last meal.

Vineet Augustine, PhD, a Scripps Research Fellow who works closely with Patapoutian’s lab, says the discovery of PIEZOs not only explained how mechanical forces are sensed but also provides scientists an entry point to finding the neurons involved.
“One of the major challenges of modern neuroscience is to identify different types of sensory neurons and find out how they come together to process information,” says Augustine. “PIEZOs are a terrific example of this. When we find neurons that express PIEZOs, we can speculate that they might be involved in force-sensing.”
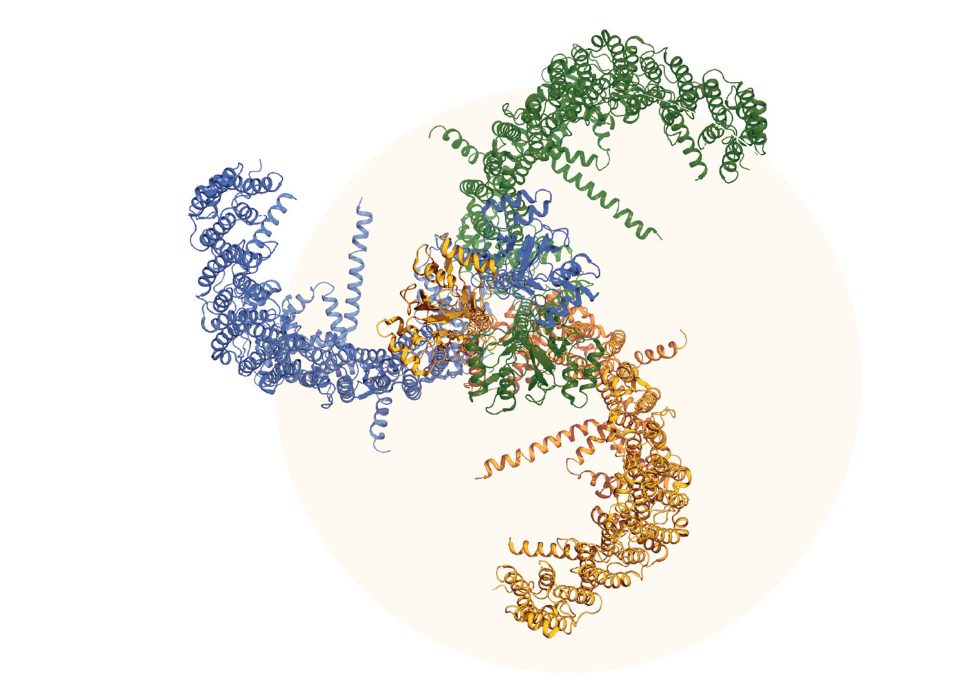
Illustration of the molecular structure of a PIEZO1 channel, a protein found in the outer membrane of some neurons and cells that allows them to sense mechanical forces. These channels are important for touch sensation as well as interoceptive sensing taking place in internal organs.
Courtesy: Ardem Patapoutian, PhD.
Identifying these sensory neurons gives researchers a place to start tracing their path back to the brain. This painstaking research maps the circuitry and computing logic that allows our bodies to maintain physiological balance and respond to our internal and external environment. In his current research, Augustine focuses on circuits that allow the heart, kidneys and brain to work together to maintain cardiovascular homeostasis, things like proper blood pressure, heart rate and blood flow, especially during physical or psychological stress.
“One challenge with studying interoceptive systems involved in diseases is that there’s often not one underlying cause,” Augustine says. “For example, with high blood pressure, there are often changes in kidney function, changes to the heart and changes to the blood vessels themselves.”
He notes that some cardiovascular patients don’t respond to existing therapies for high blood pressure, possibly because the drugs don’t target the precise underlying causes. It could be that the problem is due to some miscommunication between the brain and a certain organ, and if scientists can decode those circuits, they might be able to develop drugs that target the exact cause.
Neuroscience Technology Renaissance
Augustine says one of the reasons interoception as a field of study lagged behind research on external senses (collectively known as “exteroception”) was because research on different organs was siloed into separate fields of physiology. Cardiologists studied the heart, nephrologists the kidneys, and so on. Neuroscientists, for their part, were focused on external senses which were more accessible for study, being located on the outside of the body.
The recent attention given to interoception stems from mounting evidence that communication between internal organs and the brain—and disruption of that dialogue—may play a central role in many diseases. This dovetailed with rapid advances in the technologies available for mapping the nervous system and deciphering its programming and computing logic. For example, a technology known as “optogenetics” allows researchers to turn specific neurons on and off using flashes of light, so they can discern their various roles. Modified forms of various viruses, such as rabies and herpes, allow Augustine and others to trace connections between neurons and to visualize the architecture of neural circuits as never before. A new method known as tissue clearing essentially makes the brain and other organs transparent, so that researchers can now observe neuronal architecture in an intact organ. And using calcium imaging, another optical technology, they can monitor real-time neuronal activity.

Li Ye, PhD, an assistant professor of neuroscience at Scripps Research, has pioneered the use of tissue clearing to study the neurons that innervate the body’s fat tissues. “People long thought fat was just for energy storage,” says Ye, “but this notion has been dispelled by the realization that our fat communicates with the rest of our body, including through sensory neurons that carry information to the brain.”
Our brains appear to keep track of where and how much fat we have stored in our bodies. While still a matter of debate, there is some evidence that based on signals coming from our fat tissues, the brain may establish a “set point” for where and how much fat we store. Even if fat is removed, due to surgery or dietary changes, the brain may tend to alter our physiology and behavior to regain fat to return to that set point.

“We share many genes and neurological mechanisms with C. elegans, so as we unfold these systems in worms, we’ll be better positioned to understand the impact of interoception in humans.”
-Supriya Srinivasan, PhD
In a State: Worms, Fat and Rodent Romance
Supriya Srinivasan, PhD, an associate professor of neuroscience at Scripps Research, says that while the set point concept in fat metabolism is still theoretical, it conveys a core concept of interoception: namely, that our brains use signals coming from our integral organs to establish overarching “states” that influence our physiology, behavior and mental condition.
Srinivasan offers the example of the reproductive behavior of the microscopic roundworm C. elegans, a species her lab studies and a common model organism used in neuroscience. For the worms to reproduce, they need to have stored enough fat, which they stow in their intestine, and they must be in an oxygen- and food-rich environment.
Sensors in a worm’s intestine are responsible for tracking its fat stores and external sensory neurons keep track of the amount of oxygen and food in the environment, as well as the social situation—how many other worms are around, for instance. If you remove the fat from a worm’s intestine, not only will the worm not reproduce, but the intestinal sensor sends a chemical message that tunes the external sensory neurons to the diminished fat status. Put another way, the worm’s gut, having sensed fat stores are low, puts the worm into a state where it can no longer respond to external cues. Deciphering this dynamic in worms can tell us a lot about ourselves.
“At the moment, we’re trying to map out the intestinal sensors and the chemical signals of a single tight circuit,” says Srinivasan. “But we also want to know what other neurons are paying attention to what’s happening in the gut. It could turn out that signals coming from the intestine could be influencing other aspects of the worm’s physiology and behavior. We share many genes and neurological mechanisms with C. elegans, so as we unfold these systems in worms, we’ll be better positioned to understand the impact of interoception in humans.”

“It’s becoming clear that what’s going on inside your body is as important as what’s going on in your environment. Your brain is integrating information from outside and inside to figure out what it should do in every moment.”
-Lisa Stowers, PhD
Lisa Stowers, PhD, a Scripps Research professor of neuroscience, says there are clear examples of interoceptive signaling that influence the behavior of mammals. Take, for instance, rodent romance. Male mice produce pheromones to entice females to mate with them. Females sense these chemicals l’amour via neurons in their noses. However, these neurons only respond to the pheromones when the female is in estrous and therefore able to conceive. Here again, we have an internal state influencing how an organism’s nervous system interacts with the external world and its behavior.
Stowers is working to identify the areas of the mammalian brain responsible for receiving and processing such interoceptive information. In addition, she’s hoping to decipher how interoceptive signals from different parts of the body combine in the brain with information coming from external senses, such as sight, hearing, taste, smell and touch.
“A big chunk of our brain is dedicated to monitoring what’s happening inside—what state you’re in, whether you are agitated or whether you’re calm. Are you hungry, are you thirsty, are you tired,” Stowers says. “It’s becoming clear that what’s going on inside your body is as important as what’s going on in your environment. Your brain is integrating information from outside and inside to figure out what it should do in every moment.”
To unravel how the brain integrates interoceptive signals with high-level behavioral control, Stowers’ team is focused on an unglamorous body function: urination. At first glance, urination seems like a purely visceral function, but the brain and peripheral nervous system are integrally involved. In fact, potty training is one of the earliest socially dictated behaviors children learn—a skill that any parent of a toddler can tell you is no easy feat to master. To avoid a wet diaper, children must first learn to recognize the sensations coming from their bladder and break away from Baby Einstein videos, a favorite toy or whatever other object in their environment currently holds their attention. Then they must head, in a timely fashion, for the nearest facility. Going potty, it turns out, is a microcosm of macroscale interoceptive processes. Internal molecular sensors (using Patapoutian’s PIEZO channels) send signals to the brain telling it that the bladder is full. The brain, integrating this interoceptive signal with other internal and external sensory information, must pay attention to the message from the bladder and then initiate a behavior.
As you can imagine, studying the brain-bladder interplay in human toddlers poses myriad difficulties, but Stowers’ team found a convenient stand-in—the mouse pheromone system. The pheromones a mouse uses to communicate with other mice are excreted in their urine with the intention of eliciting a desired response. Thus, a social behavior coded in the brain relies on neurological circuits connected to the bladder.
“Mice learn what other animals are doing from the smells coming from their external environment and, likewise, communicate back by emitting their own chemical signals,” says Stowers. “We can discern between when they are just reflexively urinating or when they are controlling it as part of a social interaction—which is more akin to what we do when we are actively looking for a restroom.”
By distinguishing what’s going on in a mouse’s nervous system during urination versus during social signaling, Stowers’ lab is mapping the different brain activity, neural circuits and neurons involved. Charting these systems in mice could well help pave the way to addressing incontinence associated with neurological disorders and aging. While many cases of incontinence stem from childbirth or illnesses or injury, the disorder also results from age-related neurodegenerative processes that disrupt signals between bladder and brain.
More broadly, Stowers and the other Scripps Research scientists hope to expand on their work on urination and other specific internal organ systems to discern general rules about how the body’s internal states influence higher level brain functions such as attention, social behavior and emotions. The urgency that comes with needing to urinate, for instance, might tell scientists a lot about how communications between the body’s organs and the brain play a role in anxiety disorders and depression.
If you think about it, there are a lot of situations in life where the body and brain communicate but you may not have control over it. Many people experience this during public speaking as their heart rate climbs, their palms sweat and the pitch of their voice goes up. In this case, the brain is sending a fight-or-flight alarm to the body. Likewise, our body sends signals to the brain that may contribute to emotional states that can become pathological.
“There’s growing evidence that what’s going on inside the body has much more influence on mood, emotions and behavior than we previously thought,” Stowers says. “The more we learn about interoception, the better position we are in to potentially prevent or treat related neurological disorders.”