THE FUTURE OF HEALTHY AGING
You are only as old as you think you are. At least, that’s how the saying goes. But what does it mean to age, anyway?
Increasingly, science has some surprising answers— and some promising solutions.
At Scripps Research, scientists from a range of fields are rapidly unraveling the mysteries of biological aging. They are exploring the interplay of a person’s genetic inheritance, lifestyle and environment, and how these factors influence mental and physical wellbeing as we age.
As they discover more about what undermines our health as we get older, researchers are identifying ways to help us head off these risks and stay healthy longer. In some cases, these are lifestyle and medical interventions that could apply to anyone. In others, they are therapies targeted to specific subsets of the population, for instance, people who have genetic mutations that raise their risk of certain age-related diseases.
In this feature, we will explore some of our labs’ most exciting research, from advances in understanding how aging cells disrupt our metabolism to experimental drugs designed to prevent and treat arthritis and heart disease.
The science of healthy aging is moving at warp speed with the goal of slowing down the march of biological time.
CELLULAR SECRETS TO A LONGER LIFE
Recent findings from the lab inspire new approaches to healthy aging
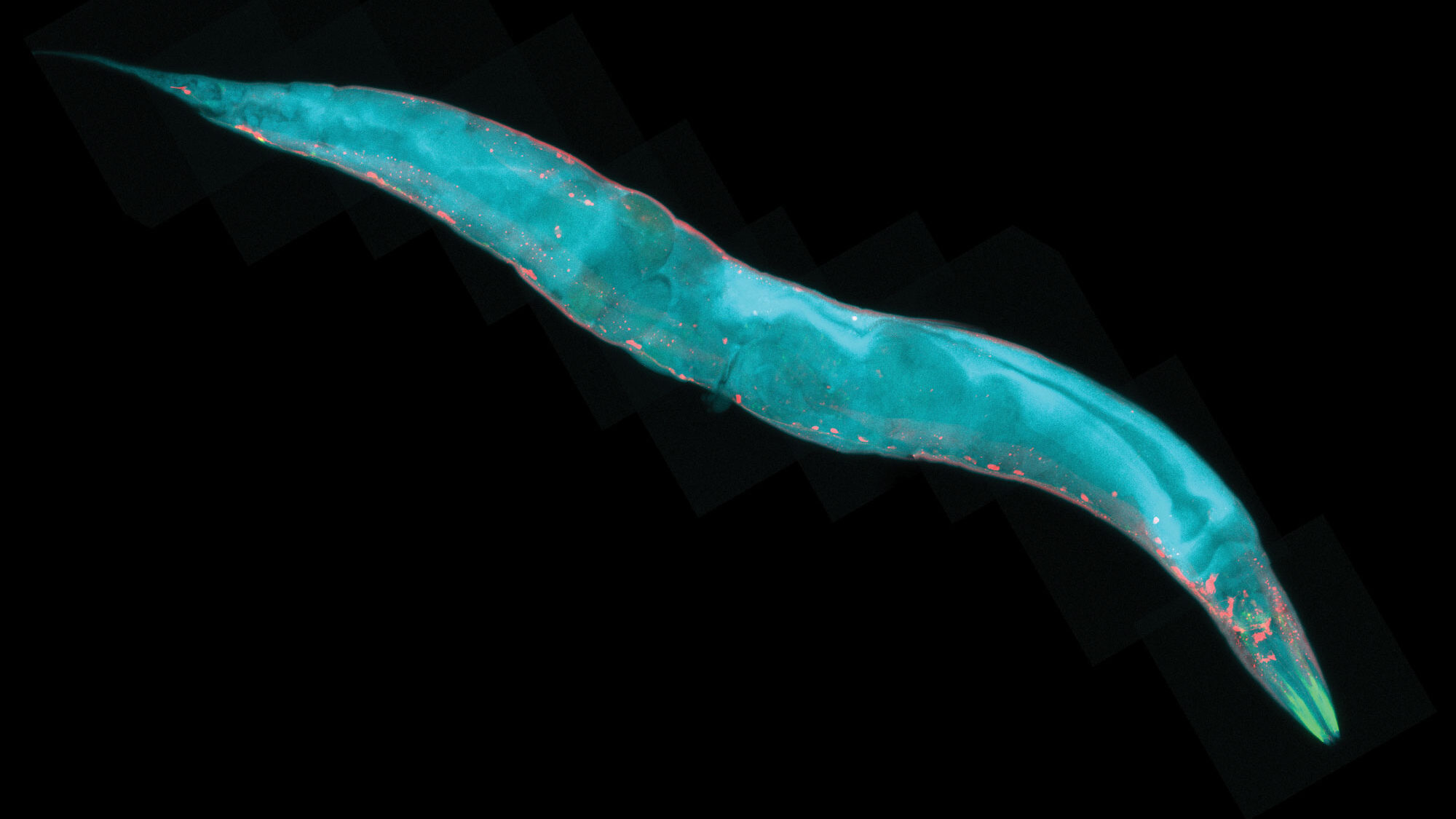
Many experiments related to lifespan involve tiny roundworms known as Caenorhabditis elegans, or C. elegans. Though the worms are only about 1 millimeter long, much of their genome is also seen in humans, performing the same functions—including genes that control metabolism, the gut and the nervous system—making it an ideal animal for longevity studies. Due to the organism’s small size, it’s possible to perform genetic screening experiments quickly and precisely, keying in on individual genes and their functions.
The Fountain of Youth is just a myth, but the scientific search for clues on how to extend the healthy lifespan of humans is very real. In the laboratories of Scripps Research in California and Florida, a swell of important findings over the past year has shed new light on how our cells and organs work together to influence longevity.
Scientists have found links to factors such as body temperature, calorie intake, diet and insulin levels. And critically, they’ve also identified ways that drugs may be able to target cellular pathways to block damaging effects or mimic the life-enhancing benefits they’ve witnessed in experiments.
Fewer calories, longer life?
Late last year, the lab of Professor Bruno Conti, PhD, made a chilling discovery—chilling in a good way. In his years studying how and why calorie restriction leads to longer life with less disease, one observation is consistent: when mammals consume less food, their body temperature drops.
“It’s evolution’s way of helping us conserve energy until food is available again,” Conti says. “It makes sense when you consider that up to half of what we eat every day is turned into energy simply to maintain our core body temperature.”
Conti’s previous work showed that lower body temperatures can increase lifespan even without calorie restriction—and that these effects involve key cellular processes, most of which were still unknown. In the new study, published in Science Advances, he and his team designed an experiment with mice that would allow them to independently evaluate the effects of reduced nutrients and those of body temperature.
“The data we collected showed that temperature has an equal or greater effect than nutrients on metabolism during calorie restriction,” says Conti, whose lab worked closely with Gary Siuzdak, PhD, chemistry professor and senior director of the Scripps Center for Metabolomics.
Together, the teams created the first comprehensive profile of metabolites—chemicals released by the metabolism—that are changed when body temperature drops. In a separate experiment, they also showed it’s possible to administer certain metabolites as a drug to change body temperature.
The teams are now advancing this work through additional experiments, with the goal of developing medicines Conti calls “temperature mimetics,” which would confer healthspan benefits without a cool body temperature.
Solving a metabolic mystery
Across campus from Conti, Supriya Srinivasan, PhD, an associate professor in the Department of Neuroscience, has performed intricate metabolism experiments with tiny roundworms to fill a knowledge gap in the longevity field. Through this, she discovered a protein that acts as a switch to protect cells from becoming overextended during times of prolonged fat-burning, such as what you might experience on a ketogenic diet.
Srinivasan is working to translate her findings into medicine that can be used to treat metabolic diseases such as diabetes or fatty liver disease.
“I set out wanting to understand this paradox that exists in longevity research,” she says. “Scientific evidence points to lifespan benefits from slowing down the metabolism through calorie restriction. But you also see benefits of speeding up the metabolism through sustained fat-burning, though mostly in context of obesity or diabetes. Nobody had looked at what happens if you sustain fat oxidation in a normal, healthy organism.”
Srinivasan built upon prior research that established a communication pathway between the brain and cells lining the intestine—the major seat of metabolic and longevity regulation. In response to information from the environment, neurons secrete a peptide that activates a receptor in intestinal cells to drive fat loss.
In her latest study, published in the journal eLife, Srinivasan found a new cellular feedback loop that’s activated only in times of prolonged fat-burning to protect longevity. Without it, sustained fat loss would trigger a damaging stress response in cells and reduce lifespan. The findings don’t just solve a mystery of metabolism research, but also inspire a new way to treat diseases that can significantly reduce lifespan.
Insulin and aging
The Florida-based laboratory of Associate Professor Matthew Gill, PhD, made a discovery in 2020 with major ramifications for longevity research. The study focused on insulin, a hormone that tells cells to pull in glucose from the blood. This helps maintain energy and keeps blood sugar in a safe range.
For decades, researchers have recognized that insulin signaling is also an important regulator of longevity. Unregulated signaling has shown to reduce lifespan, while reduced signaling can extend lifespan. In experiments with the C. elegans worm, a gene mutation that lowers insulin signaling can more than double the worm’s lifespan.
In Gill’s recent study, published in eLife, he and his team discovered in roundworms a “decoy” insulin receptor that’s essentially a truncated version of the normal receptor, DAF-2. The decoy, which they named DAF-2B, is secreted into the area surrounding the tissues of the worm, capturing insulin molecules before they can complete their signaling task.
The scientists found that overproducing the decoy tips the worms into a semi-dormant state that normally occurs when food is scarce. Overproduction of the decoy also increased worm lifespan.
Gill suspects that the decoy is produced at high levels early in an organism’s life, then drops off in response to aging and disease. If so—and if humans also have a decoy insulin receptor—then scientists will have a new way of acting on a key regulator of longevity. Gill is continuing his research to find out.
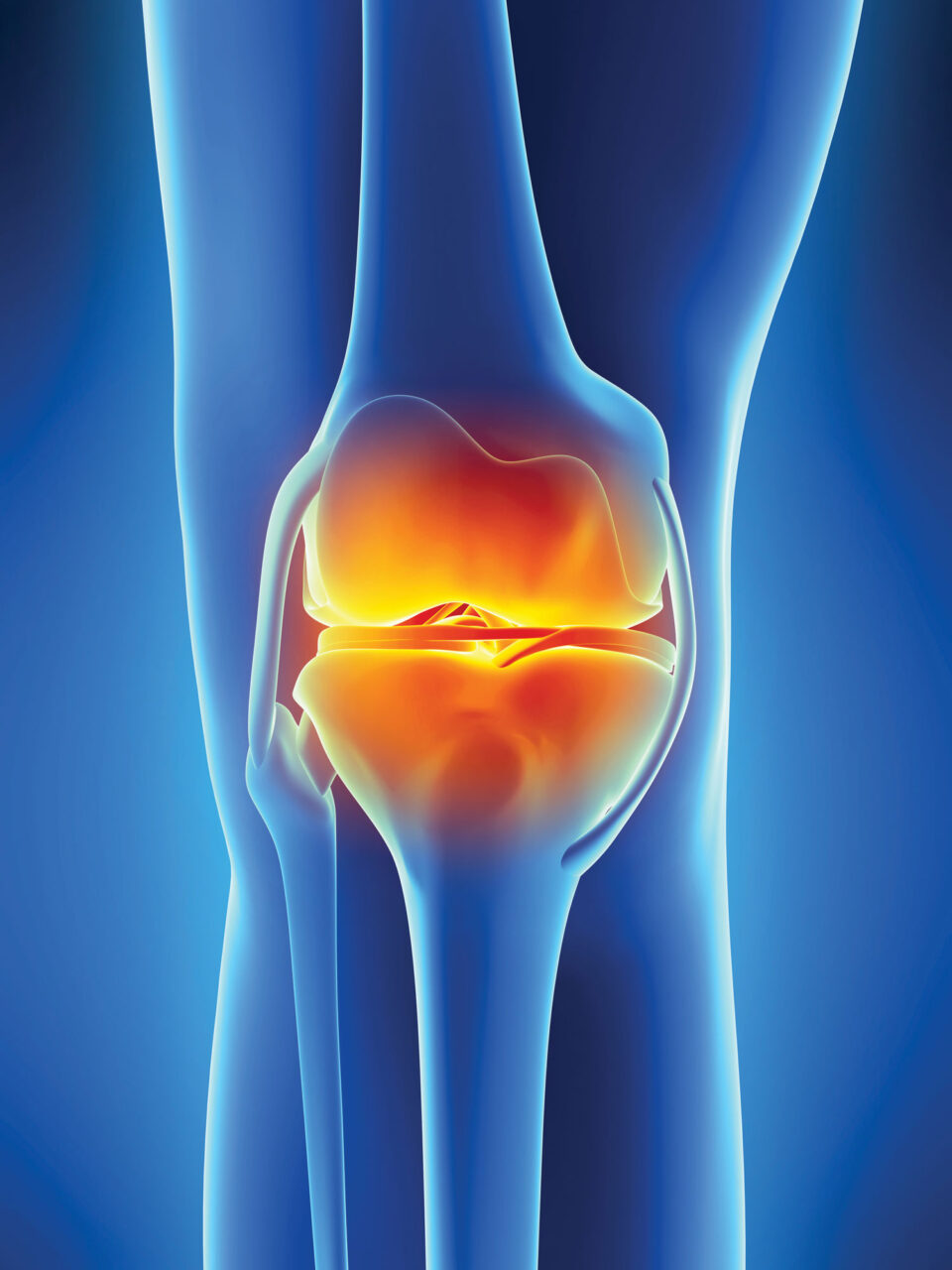
OVERCOMING ARTHRITIS
Scientists hope to remove stiff, achy joints from the aging equation
The legendary comedian Jack Benny famously said of an honor he had just received, “I don’t deserve this award, but I have arthritis, and I don’t deserve that either.”
Benny wasn’t alone in finding that, deserving or not, achy joints are common hallmarks of reaching one’s golden years, the result of cartilage breaking down due to wear-and-tear and age-related physiological changes. More than 32 million people in the United States are currently living with osteoarthritis, the form of arthritis typically associated with age-related deterioration of the joints or joint injury.
But does it have to be that way? Promising research and experimental therapies from scientists at Scripps Research suggest perhaps not. As they decipher the underlying biology of how the body’s ability to maintain and repair cartilage changes with age, they are finding a number of exciting possibilities for counteracting the onset of age-related arthritis.
“Over the past two decades, we’ve learned a great deal about what aging is at the cellular level,” says Martin Lotz, MD, a professor in the Department of Molecular Medicine at Scripps Research. “What we are finding is that there are commonalities among the various tissues of the body and that ideas for preventing degeneration in one organ or tissue may be applicable to others.”
Lotz noted, for instance, that in both neurodegenerative diseases and osteoarthritis the process of disposing of old and malfunctioning cells, known as autophagy, is thought to be disrupted in older people. As this janitorial function declines with age, cells remain in the body beyond their useful life, generating toxic inflammatory substances that damage other tissues.
In his research, Lotz has focused on several proteins that appear to play key roles in the maintenance and repair of cartilage. One intriguing area is a class of proteins called FOXO transcription factors, which are central to autophagy and involved in cancer, neurodegeneration and aging.
In recent studies, Lotz and his collaborators analyzed FOXO activity in cells from the shock-absorbing structures in the knee. These structures include the cartilage attached to the ends of the bones in joints and C-shaped pads of cartilage between the bones, known as menisci. They found that people with osteoarthritis showed abnormal FOXO activity compared to people with healthy knees. Lotz and the team at Calibr, the drug development division of Scripps Research, are developing experimental compounds for osteoarthritis that target FOXO, as well as other families of proteins. Calibr is also targeting cathepsin, a family of enzymes that play an important role in the breakdown of cartilage.
Another transcription factor known as Mohawk also appears to play an important role in joint tissues and the development of osteoarthritis. Studies by Lotz and others have shown that Mohawk’s activity is suppressed in people with osteoarthritis, suggesting that therapies that boost Mohawk activity might prevent joint degeneration or even repair damage. Adding support for that notion, Lotz and his collaborators found that administering Mohawk to the knees of mice stimulated cartilage repair.
Stem cells in the joints are another area of intense interest for researchers. Calibr recently completed a phase 1 clinical trial of KA34, a drug designed to stimulate the regeneration of cartilage from mature stem cells found in the knee. The idea for the compound emerged from the laboratory of Peter Schultz, PhD, a chemistry professor and the President of Scripps Research. Schultz and his team discovered a prototype compound, that they named kartogenin, that generates chondrocytes from stem cells, the early-stage, highly flexible cells from which all specialized cell types develop.
In a groundbreaking paper in the journal Science, Schultz and his collaborators reported that when given to animals, the compound was effective at regenerating cartilage. In the human clinical trial that wrapped up last fall, which was designed to test the basic safety of KA34, the drug was administered to 60 patients with osteoarthritis and was well tolerated. Lotz, who oversaw the trial, said there was some evidence that KA34 had effects on the disease, but quantifying its efficacy as an osteoarthritis therapy will require additional trials.
Currently, Lotz is conducting genomic analyses to better understand the biological action of KA34 in the knee tissues and is working with the researchers at Calibr to develop a slow-release formulation of the drug that would allow the compound to be released over a period of several weeks or months once injected into the knee.
“With the combination of Scripps Research and Calibr, we’re really on the trail of some very exciting leads in terms of developing the next generation of osteoarthritis drugs,” said Lotz, summing up the state of the field. “In combination with what we’re learning about other means of protecting and healing joints, including diet, exercise and other lifestyle interventions, the development of disease-modifying therapies could dramatically change the treatment of arthritis in coming years.”
CHANGING COURSE BEFORE IT’S TOO LATE
Genetic testing leads the way to better disease prevention, longer lives
Only decades ago, family history was the only way for most people to estimate their genetic risk of developing a chronic disease as they age. “It runs in the family,” is what someone might say about a propensity for heart attacks or diabetes. Then, it was a waiting game.
That changed with the advent of powerful and affordable DNA sequencing equipment, giving rise to personal genomics companies. Today, the average person has access to a far more precise way to know their innate risks for many common diseases.

Unlike rare “monogenic” diseases such as cystic fibrosis or certain cancers, which are caused by a mutation to a single gene, chronic diseases are often “polygenic,” or influenced by a large number of genetic variants that can span the genome.
In recent years, polygenic risk scores have become more accurate and clinically relevant, providing a way to alert people to their previously unknown likelihood of disease. Risks that once flew under the radar are now in the open.
At Scripps Research, scientists are digging into genetics in new ways to identify polygenic markers of health and disease—with the ultimate goal of intercepting illness or preventing it altogether.
“When you know your genetic disease risk, you become empowered to change your trajectory to live a longer and healthier life,” says Ali Torkamani, PhD, director of Genomics and Genome Informatics at Scripps Research Translational Institute. “Depending on the disease, there are different windows of opportunity for intervention.”
Torkamani has become particularly interested in the genetic factors of coronary artery disease, the leading cause of death among both men and women in the United States. Untreated, it leads to heart attacks, heart failure and sometimes sudden cardiac death.
Yet, coronary artery disease is not a major worry for most people, unlike cancer and other conditions that are far more uncommon. “If you look at what people are googling versus what actually kills us, you see that heart disease is very much overlooked, despite being the single biggest disease threat,” Torkamani says.
He and his team have launched a study called MyGeneRank, which calculates a cumulative genetic risk score by analyzing a person’s DNA and correlating it with volumes of research on patient health outcomes. Anyone with a 23andMe report can join the study via a smartphone app; so far, about 5,000 people have signed on to learn their risk scores.
Those with high genetic risk for coronary artery disease are linked up with resources for making dietary or lifestyle changes. They’re also encouraged to consult their physician about taking medications such as statins that can prevent a heart attack.
“It is very possible to compensate for a high genetic risk score with healthy habits,” Torkamani says. “The sooner you start taking action, the better, but it’s never too late.”
Even people with healthy cholesterol levels, weight and blood pressure may learn their risk is high, as the program looks at 168 genetic risk markers that relate to a variety of invisible factors, such as the elasticity of your veins.
Among participants, about 15 percent of the high-risk users have reported that they started using statins or changed their medication in light of the new knowledge.
In separate research, Torkamani and his team are also studying polygenic risk factors of people who died suddenly from cardiac issues; they’re in the process of analyzing genomes from people who have recently died in San Diego, in the hopes of finding clues that can be used for screening.
GETTING TO THE HEART OF THE MATTER
Precision therapies for genetic and age-related diseases
A person’s health as they age depends a lot on how they take care of themselves. Do they eat a healthy diet? Do they exercise regularly? Do they avoid smoking and other unhealthy habits?
Yet, as salubrious as a person’s lifestyle may be, genetics also play a critical role in their health—at times to detrimental effect.
Fortunately, as scientists unravel the ways that genetic inheritance influences the function of a person’s cells, tissues and organs, and hence their health as they age, they are also finding new ways to prevent and treat age-related genetic disorders and related sporadic diseases linked to aging.
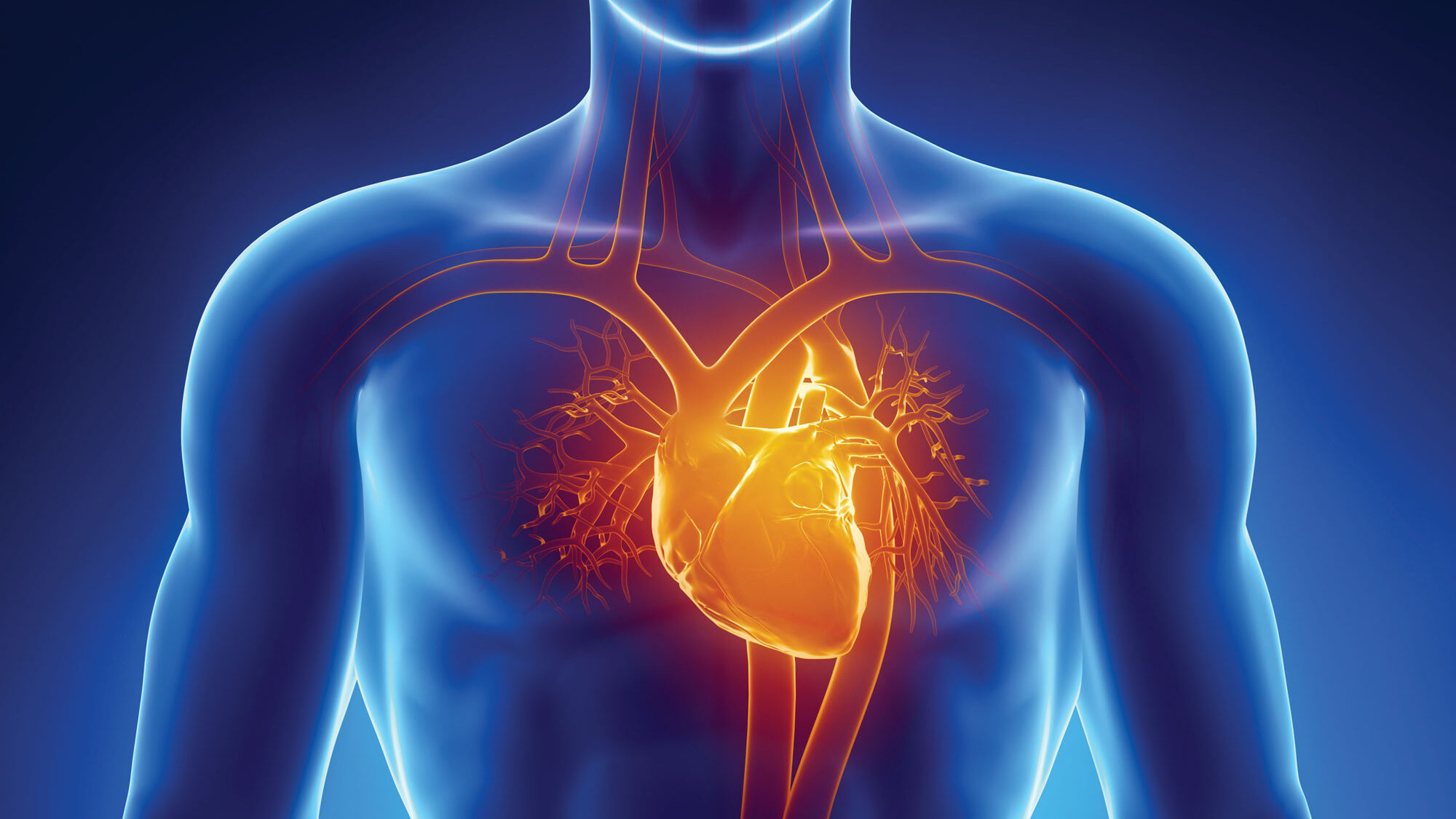
Such is the case with tafamidis, a drug discovered at Scripps Research for the treatment of transthyretin amyloid cardiomyopathy (ATTR-CM), a potentially deadly form of heart disease that most often affects men over the age of 60. If left untreated, the disease typically leads to death within 5 years. There are both genetic and sporadic forms of this aging-associated disease.
“Before we understood what was actually happening at the cellular and genetic level in patients with ATTR-CM, there was no way to effectively treat them for their particular form of heart disease,” says Jeffery Kelly, PhD, a professor in the Scripps Research Department of Chemistry who discovered tafamidis with his colleague, Evan Powers, PhD. “In this case, knowledge is power – the power to intervene in the progression of a disease that can significantly shorten people’s life.”
In patients with ATTR-CM, a protein called transthyretin, which is produced by the liver to carry thyroid hormone and vitamin A, is prone to becoming misshapen. These misfolded proteins clump together to form amyloid fibrils or amyloid plaques, which collect in heart tissues, killing cells and damaging the heart.
Sometimes linked to a genetic mutation, ATTR-CM is one manifestation of a broader set of disorders that fall under the umbrella term, “transthyretin amyloidosis,” where aberrantly folded transthyretin proteins cause amyloid plaques to form in the body. Other forms of the disease impact the nervous system, where build-up of amyloid plaques in cells of the central and peripheral nervous system lead to neurological symptoms.
Tafamidis binds to the normal shape of transthyretin which cannot form plaques, preventing the transition to the misshapen form of transthyretin which precludes transthyretin from clumping together in the first place. This stops the formation of amyloid plaques and heads off the tissue damage seen in the disease.
Tafamidis was first approved for treatment of familial amyloid polyneuropathy (FAP), a neurodegenerative disorder where transthyretin plaques form in nerves of the peripheral and central nervous system. In 2019, the U.S. Food and Drug Administration (FDA) approved two formulations of the drug, Vyndaqel (tafamidis meglumine) and Vyndamax (tafamidis) capsules, as the first FDA-approved treatments for ATTR-CM, both the genetic and sporadic forms of heart disease caused by transthyretin amyloidosis.
The FDA approved the therapies after a clinical trial found that they significantly reduced the risk of hospitalization and death for patients with an inherited form of the disease, as well as in sporadic cardiomyopathy patients who had no clear genetic link.
The development of tafamidis is a prime example of how one can stop or slow the progression of an age-related disease by precisely targeting a biological component of its underlying cause, says Kelly.
“Aging isn’t a monolith progress that impacts everyone in the same way, and we need therapies that are tailored to individuals and their specific biological progressions of aging,” says Kelly. “As our understanding of the mechanisms of age-related diseases advances, we will see more and more precision therapies that intercept a wide range of illnesses.”
STRESS, DISEASE AND THE BIOCHEMISTRY OF AGING
Chemist Kate Carroll discovers fundamental ways cells repair oxidative damage, blazing a trail for new treatments
Oxidative stress damages and ages cells in fundamental ways, says Kate Carroll, PhD, a chemistry professor at Scripps Research, Florida.
It changes the chemistry of amino acids. It garbles and unfolds proteins. It disturbs the organelles that power cells, called mitochondria, and it even causes cell death. In brain diseases like Parkinson’s, garbled protein clumps are a hallmark of the disease process.
“When you get oxidative damage, you get protein unfolding and aggregation – clumping. Then you see neurodegenerative disease,” Carroll explains.
Too little sleep, too much pollution, exposure to heavy metals, or simply time spent in the sun, and suddenly free radicals zip around cells causing havoc. That’s oxidative stress, and it results when a system is out of balance. But what are free radicals? Very simply, they’re negatively charged, unbound oxygen molecules looking for a place to settle.
Stress causes cells to age. Learning how and why is highlighting new approaches to repairing the damage, with implications for cancer treatment, Alzheimer’s, Parkinson’s and more.
Carroll has invented a toolbox of chemistry techniques that are allowing scientists around the world to trace and study these supercharged O2 free radicals in certain contexts. In the process, her peers say, Carroll’s impactful and creative chemistry has led to profound discoveries about fundamental cell biology and biochemistry, including a finding that cells have their own enzyme-based mechanism for repairing some damaged proteins.
These discoveries have made Carroll a global leader in the reduction-oxidation (redox) chemistry field, and laid the groundwork for potential new approaches to treating a number of age-related and rare hereditary diseases, says Jamie Williamson, PhD, Scripps Research executive vice president of Research and Academic Affairs.
“Kate’s work is a beautiful example of how deep chemical insights and the tools of chemistry can be used to understand a fundamental biological problem,” Williamson says. “Enzymes are the cells’ chemists, and it takes a chemist like Kate to understand how they do their jobs.”
It has long been known that cells have enzyme-based systems for repairing damaged DNA, the molecule that stores genetic information. Carroll discovered that cells have a method for repairing a large group of proteins, too, those damaged by oxidative stress. She found they rely on an enzyme called sulfiredoxin to accomplish this. Studies of the impact of sulfiredoxin on cellular health indicate it can repair and restore a wide swath of proteins and biological processes, she says.
“This is not a boutique enzyme, it is a fundamental enzyme that repairs oxidative damage to proteins,” Carroll says. Ben Shen, PhD, professor and co-chair of the Scripps Research Chemistry Department in Florida, says Carroll’s work has transformed her field.
“The innovative tools invented by Kate enabled her to make insightful discoveries that have fundamentally transformed our understanding of redox processes in cell signaling,” Shen says.
At the core of her work is the protein building block cysteine, one of 20 amino acids that make up proteins. In their search for a stable place to land, O2 free radicals often bind with cysteine. Cysteine is an outlier among amino acids for all the sulfur molecules it carries, making it the preferred destination for free radicals. But as those sulfur molecules soak up the O2, they are changed in the process. That sets off a chain of impacts that can lead to disease.
Carroll hopes to see these insights used to engineer new types of medicines designed to heal oxidative damage to cells. An important first step toward that goal is understanding when hyper-oxidized cysteine carries out a beneficial, helpful role, as opposed to promoting disease states.
Carroll is now collaborating with a group at Harvard University, led by T. Keith Blackwell, PhD, to study these processes in nematodes, a type of roundworm used to study basic biology. The data are demonstrating that cells are heavily impacted by hyper-oxidized cysteines, Carroll says.
To arrive at these many insights, Carroll had to develop chemistry tools that allow selective study of oxidation-damaged proteins. With these tools, she can work on development of drug-like compounds to address a variety of currently untreatable diseases. The compound she’s currently developing moves to cells’ mitochondria, where the free radicals are being produced, and sequesters reactive oxygen species before they can cause damage.
“The compound senses the location of oxidative stress damage in the cell and mitigates it,” Carroll says.
Diseases of aging are one area of potential application, she says. There are also a number of rare, hereditary genetic disorders that cause mitochondria to malfunction, and that’s where Carroll sees these compounds having an impact first.
“Their mitochondria don’t produce enough energy for the body to function properly, so you have children who don’t grow well, they have muscle weakness, cognitive effects,” Carroll says. “There are no treatments. None. They affect on the order of 25,000 children a year.”
THE AGE OF
EMPOWERMENT
Growing older comes with a number of major life changes: career transitions or retirement, the prospect of grandchildren, and in particular, the potential for significant physical and mental health challenges. Indeed, a growing concern for both individuals and society is that the continual increase in lifespan is not frequently paralleled by a similar increase in healthspan.
Just as there are some exciting new drugs and technologies in development to prevent and reverse age-related conditions, science also helps to inform the things we can do in the here and now to forestall the most serious features of the aging self. Awareness of these biological processes is the first step to taking positive actions that could alter the trajectory of our later years and transform our notion of inescapable aging into healthy maturation.
ADVANCE STRENGTH
As we age, we tend to lose muscle mass (sarcopenia) and bone density (osteoporosis), which can reduce our mobility, make us less resilient to physical injuries and place us at higher risk for serious fractures. Indeed, maintaining muscle and strength is greatly associated with improved healthspan and lifespan, even independently of body fat levels or cardiovascular risk factors. Beyond simply movement, muscle tissue is now known to release its own chemicals called myokines, which circulate around the body and may have several beneficial activities, ranging from improved cognitive health, to greater immunity and even anti-tumor effects. The best way to promote musculoskeletal health throughout life is by regular exercise, particularly resistance-based exercise that prioritizes strength and recruits a significant amount of muscle.

A BRIGHT FUTURE
Vitamin D, the sunshine vitamin! Despite the name, vitamin D is in fact a critical hormone. It performs important roles all over the body, such as controlling calcium levels to maintain healthy bones, improving the cardiovascular system and boosting our immune function. Vitamin D is best acquired from getting out in the sunshine, where it is produced in the skin from exposure to solar UV rays. Modest amounts can also be found in oily fish, eggs, and some dietary supplements. As we approach our advanced years, the risk of vitamin D deficiency significantly increases as the conversion process in the skin becomes less efficient and we tend to spend less time outdoors. This risk increases further with obesity, as vitamin D can stay trapped in body fat deposits and is less able to reach the rest of our tissues.
GETTING OLDER, STAYING WISER
What if you couldn’t remember which day it was or recognize the face of your dearest loved ones? One of the most unsettling aspects of aging is the potential for neurodegenerative disease and cognitive decline. In later life our brains shrink in volume, which is attributed to the loss of neurons and a reduction in their active connections. There is also a greater risk for developing plaques in the brain, which is thought to underlie forms of dementia such as Alzheimer’s disease. These neurodegenerative conditions are increasingly prevalent in those with diabetes, suggesting that the brain’s blood flow and ability to use sugar for energy may be compromised. Although scientists are still exploring the true origins of neurodegeneration and potential medicines to combat them, research indicates that intellectually engaging activities, a healthy Mediterranean diet and regular physical activity could at least slow the rate of age-related decline.

GOING WITH THE FLOW
Many of us know older family members and friends who suffer from some form of cardiovascular disease, a debilitating condition that remains the number one killer in the United States. With age, our major arteries can become thicker and less flexible, causing an increase in blood pressure, and placing undue stress on the heart muscle. These effects can be worsened by chronic inflammation arising from obesity, smoking and the long-term consumption of processed foods. As well as modifications to diet, exercise and sleep, managing stress may be a key factor for the aging heart. When we’re overwhelmed, we release “stress hormones” such as cortisol and adrenaline, which spike blood pressure and increase our heart rate. A regular mindfulness practice such as meditation or yoga has been shown to release us from this “fight-or-flight” state, improve blood flow and reduce the risk of heart attack and stroke.

RELATIONSHIP GOALS
Can our friends make us live longer? As social animals, maintaining a strong sense of community and close personal relationships into old age could be an underestimated contributor to longevity. Some research suggests that the social isolation of seniors results in cognitive and physical decline, increased infection rates and in the worst cases, early death. Different psychological theories exist as to why maintaining close relationships is beneficial to our health. One possibility is that a partner, along with family members and friends, offers strong emotional support that can help an individual overcome periods of high stress. Another theory suggests that those closest to an individual may directly encourage them to make behavioral changes that positively impact their health, such as alternate lifestyle choices and seeking medical attention when illness strikes.

FUELING THE ENGINE
“My metabolism is slowing down!” Why does it seem so much easier to put on those pounds as we get older? Aside from being less active behaviorally, the aging body is internally less effective at growing and replacing its tissues, meaning we expend less energy. A major component of this change is the loss of lean muscle mass with age, which normally accounts for at least 20 percent of our resting metabolic rate. This energy-hungry organ system is also best at taking up and using sugar after a meal. When muscles shrink and their function declines, we lose the ability to tolerate sugar, which can increase the risk of becoming obese, insulin resistant and developing type 2 diabetes. This often occurs side-by-side with age-related changes in important hormones, such as testosterone in men and estrogen in women, which normally promote metabolic health, as well as overall vitality.