If you thought your 5G network was fast, consider that a single neuron in the human brain may send up to 200 signals every second. And with approximately 86 billion neurons in the brain, our heads contain the most complex communications network imaginable.
However, the complexity and interconnectedness of the brain also present challenges in health and medicine: when parts of the network fail, developmental disorders, mental health issues and neurodegenerative disease can result.
From the genetic underpinnings to visible behavioral outputs, Scripps Research scientists explore every part of how nerve impulses are transmitted and explore molecular approaches that could prevent, treat or even reverse conditions of the nervous system.
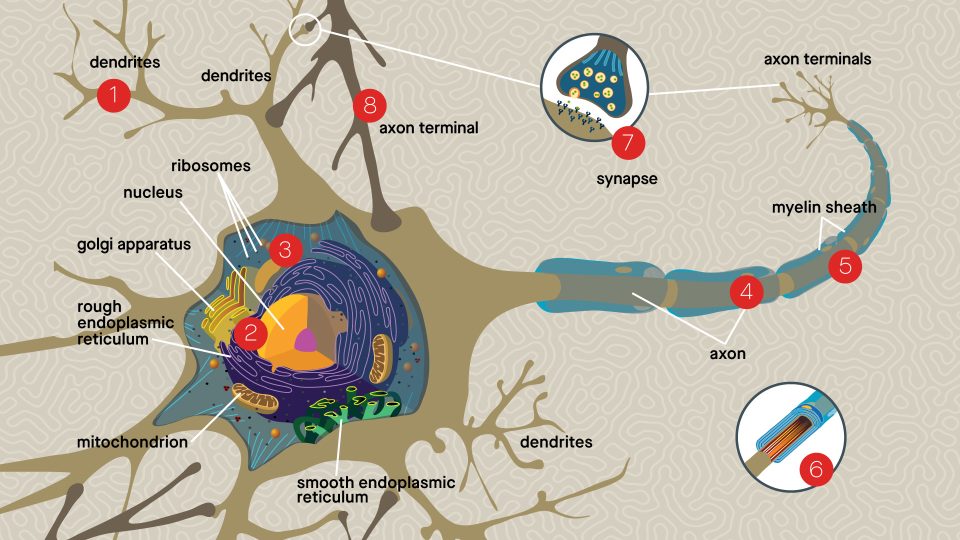
Dendrites
Tree-like structures receive electrical impulses from neighboring neurons. These impulses are then transmitted along the axon to the other end of the neuron, to propagate the signal.

Cutting-edge imaging of dendrites display a connectivity map of brain circuits. By understanding the balance of electrical inputs to collections of neurons, scientists like Hollis Cline, PhD, are revealing which defects in the visual system contribute to eye disease and how connectivity changes can result in behavioral disorders.
Cell Body
The core section of the neuron contains its genetic information and carries out the energy, protein and neurotransmitter production required for cellular activities.

Sophisticated genetic engineering technologies used by Xin Jin, PhD, identify how various mutations impact brain development and contribute to autism spectrum disorder. Discoveries by Xiang-Lei Yang, PhD, pinpoint an ancient family of proteins that connect DNA with the wider cellular environment, helping to advance potential gene therapies for rare, inherited neurological diseases.

Scientists led by Michael Petrascheck, PhD, have determined that by putting the brakes on ribosomes, the molecular factories that build proteins, the synthesis of certain error-prone proteins can be slowed, reducing the chance of plaque formation characteristic of Alzheimer’s disease.
Axon
If the cell body is the city, the axon is the connecting road which traffics all the important molecular components to the rest of the neuron. The axon is insulated with a specialized fatty layer called the myelin sheath, which allows electrical impulses to transmit quickly and efficiently along the nerve cells.

In multiple sclerosis (MS), the immune system mistakenly targets the myelin sheath, disrupting the flow of information within the brain. A first-in-class therapy called ozanimod, developed by Scripps Research’s Hugh Rosen, MD, PhD, and Ed Roberts, PhD, works to redirect immune cells away from the myelin sheath, preventing the autoimmune attack and reducing disease progression in patients with relapsing forms of MS.

Luke Lairson, PhD, and colleagues are developing drugs that can regenerate the myelin sheath that has been lost during MS or other inflammatory diseases of the brain and spinal cord. The novel molecules instruct the brain to make new oligodendrocytes—the nearby cells responsible for producing the myelin sheath. Adding these compounds to a therapeutic regime could help spark the process of remyelination to help repair damaged nerve cells.

By studying how nanomachines inside the axon move valuable cellular cargo along molecular tracks, Sandra Encalada, PhD, is exploring how neuronal trafficking is critical for the process of learning and memory. The breakdown of this transport system can lead to traffic jams inside of neurons, whereby proteins misfold and form the toxic aggregates seen in Parkinson’s and Alzheimer’s disease, as well as prion diseases like Creutzfeldt-Jakob Disease (CJD).
In collaboration with Jeff Kelly, PhD, Encalada has identified chemical agents that can break down these aggregates, remove the blockage and restore neuronal health. These candidates are now in preclinical models of neurodegenerative disease, while a similar molecule, tafamidis, developed in Kelly’s lab, is already approved for clinical use for an inherited form of peripheral nerve disease.
Axon Terminals
Button-like endings of the axon make connections, or synapses, with other neurons or target cells in close proximity. Neurotransmitters are released from the terminals and move across the synapse to relay signals to the next cell.

Breakthrough research on psychological stress by Hugh Rosen, MD, PhD, has implicated a role for types of opioid receptors involved in emotional control. Clinical scientists led by Rosen are testing a medicine that finely tunes these receptors to reduce the stress response and ease the symptoms of depression and anxiety.

Destruction of synapses and loss of brain connectivity are some of the hallmark features of neurodegeneration. Experimental therapies tested in human “mini-brain” models of Alzheimer’s, called cerebral organoids, are showing success in stopping excessive electrical activity that can ultimately cause the loss of brain synapses. Additional promising studies by Stuart Lipton, MD, PhD, demonstrate that these molecules can also promote regrowth of lost synapses, which could restore cognition and halt disease progression.