Fluorescent microscopy imaging demonstrates the anti-tumor activity of the switchable CAR-T cell platform.
Calibr, a division of Scripps Research focused on the “bench to bedside” development of transformative medicines, presented preliminary data on the development of a novel switchable CAR-T cell platform for patients with relapsed/refractory lymphoma and leukemia at the 63rd Annual ASH meeting in December 2021.
CLBR001 + SWI019 is the first clinical demonstration of Calibr’s novel “switchable” CAR-T cell platform technology, in which the activity of a universal CAR-T cell product (CLBR001) is controlled through dosage of an antibody-based molecule targeting CD19 referred to as a “switch” (SWI019). The approach represents a paradigm shift from conventional CAR-T cell therapy in that the activity of the CAR-T cells can be switched on or off through dosing of the switch to the patient. This type of switchable control over the cells is expected to improve safety and T cell durability of the therapy. Importantly, the CLBR001 switchable CAR-T is universal; by changing the switch, the CAR-T cells can be programmed to attack different antigen targets and thereby treat multiple cancers.
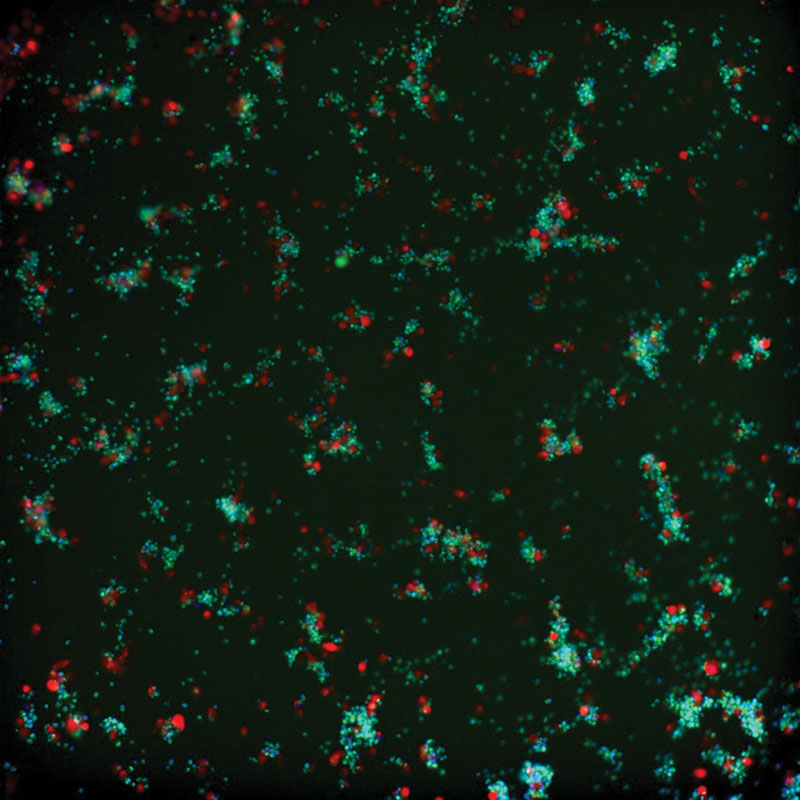
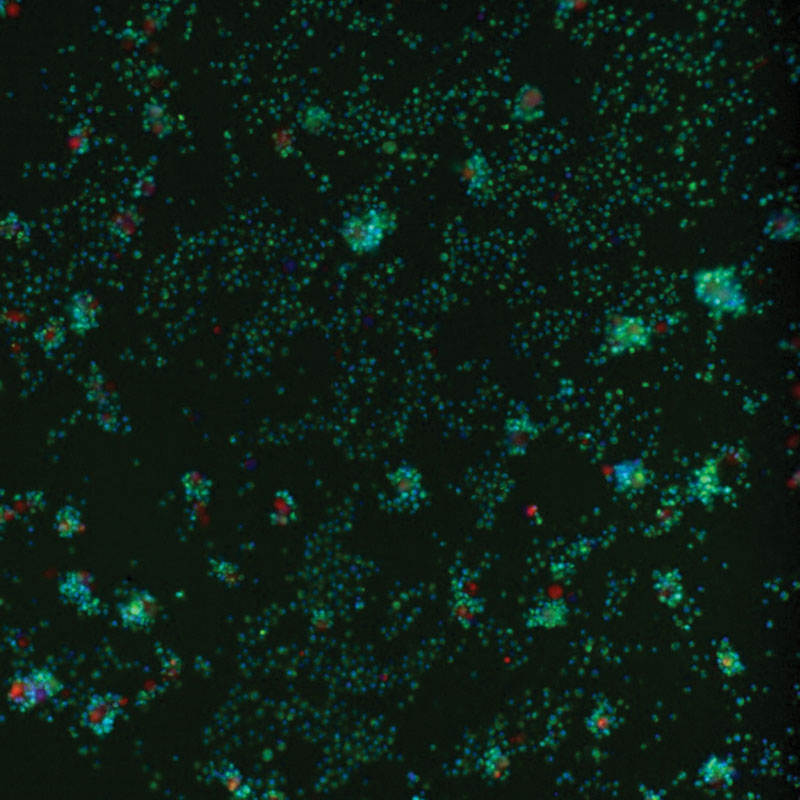
“We are very excited by initial signs of activity even at the lowest doses of CLBR001 and SWI019,” says Travis Young, PhD, Calibr’s vice president of biologics and lead on the CAR-T development at Calibr. “We believe this underscores the potential of this therapy to not only benefit patients with blood cancers, but patients with solid tumors as well.”
Calibr has partnered the switchable CAR-T cell platform with AbbVie and together they are collaborating on developing the switchable CAR-T cell platform for solid tumor indications. AbbVie holds certain rights to commercialization.