Scripps Research scientists develop super-powered vaccines and therapeutics against HIV, flu and other deadly viruses
Ever wonder why flu vaccines don’t always protect you from the flu? Or why more than three decades after the cause of AIDS was first identified, there is still no approved vaccine for HIV?
The simple answer: some viruses are especially tricky. Their genomes evolve incessantly, making them slippery targets. Like criminals donning disguises to escape the police, certain viruses can rapidly change their appearance, evading detection and eradication. Now, however, science is closing in on them.

The hub of a global network of collaborators, scientists at Scripps Research are designing and testing a new class of vaccines and drugs to defeat these evasive pathogens. Their strategy is based on a class of immune system proteins called broadly neutralizing antibodies (bnAbs) that possess a kind of superpower: the ability to inactivate a remarkably wide range of virus strains. The ability stems from the property of bnAbs to recognize the more stable regions of the outer envelope, the exposed portion of viruses that is typically targeted by antibodies.
This past year has marked several milestones in the pursuit of these “universal” therapies. Last fall, for instance, Scripps Research scientists reported engineering a prototype of a broadly-neutralizing flu therapy that protected mice from multiple strains of influenza known to affect humans. And in the past six months, the International AIDS Vaccine Initiative (IAVI) launched two clinical trials to test vaccines developed by Scripps Research scientists and their collaborators.
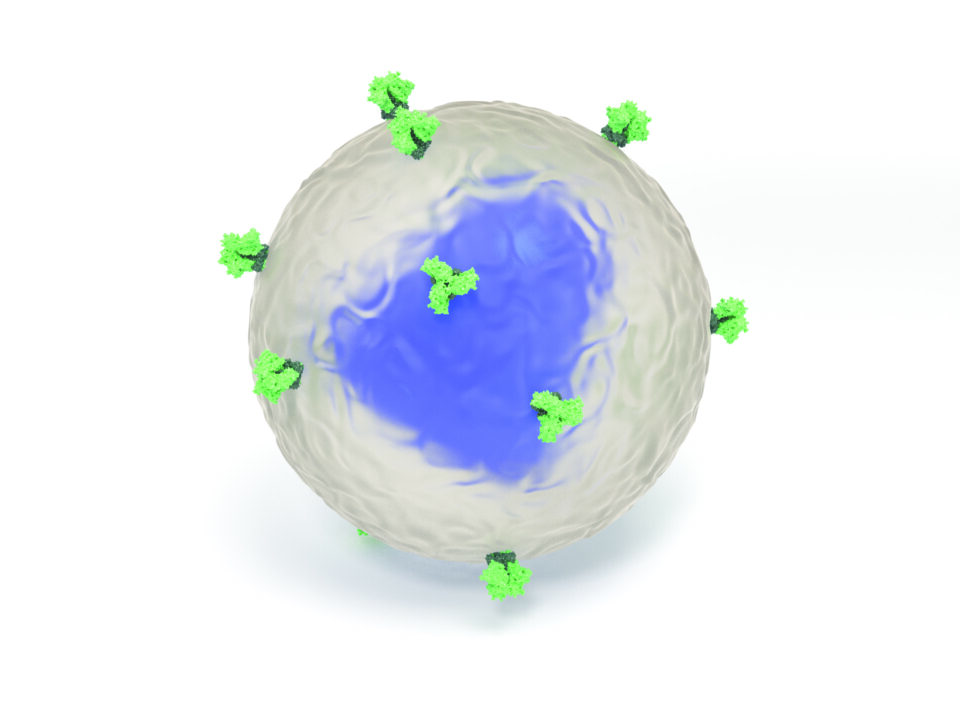
This computer-generated illustration shows an HIV virion with envelope spikes (light green) protruding from its surface (semi-transparent). The envelope spikes allow the virion to attach itself to target cells and to insert its genetic material, contained in a chamber called the capsid (blue), into them. Illustration courtesy of Lars Hangartner.
“In the past, much of the interaction between vaccines, the immune system and pathogens was invisible to us. Now we can observe these interactions in detail—at the subatomic level, in some cases.”
—Andrew Ward, PhD, Scripps Research
“We’ve reached a watershed moment in the field of immunology, where decades of research are now coming to fruition in experimental vaccines and drugs,” says Dennis Burton, PhD, co-chair of the Department of Immunology and Microbiology at Scripps Research and head of the Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery, a national research consortium supported by the National Institute for Allergy and Infectious Disease of the National Institutes of Health. “If these strategies prove effective in humans, they will represent a leap forward in our ability to manage and prevent the spread of infectious disease.”
Devious viruses meet powerful antibodies
Vaccines typically work by exposing the immune system to weakened or inactivated viruses, or to just a portion of a virus. When the vaccine is administered to a person, it lacks the ability to cause illness but retains enough of the virus’s original shape and content— collectively referred to as “antigens”—that the body recognizes it as foreign. To fight the virus, white blood cells produce antibodies capable of binding to it at certain locations, known as epitopes. If a person is later exposed to the live virus, the immune system then “remembers” the vaccine encounter and quickly ramps up its defenses to vanquish the invader.
Unfortunately, not all viruses are readily managed. Flu, HIV and other rapidly mutating viruses present a daunting challenge, as their genomes continually evolve. Thus, the epitopes that antibodies target to keep us free from infection are also ever changing.
“Scientists were able to develop effective vaccines against polio, smallpox and measles in large part because the neutralizing epitopes on those viruses are relatively stable,” says Ian Wilson, DPhil, chair of the Department of Integrative Structural and Computational Biology at Scripps Research. “But HIV and influenza mutate rapidly, so classic approaches result in vaccines that are quickly outpaced by viral evolution.”
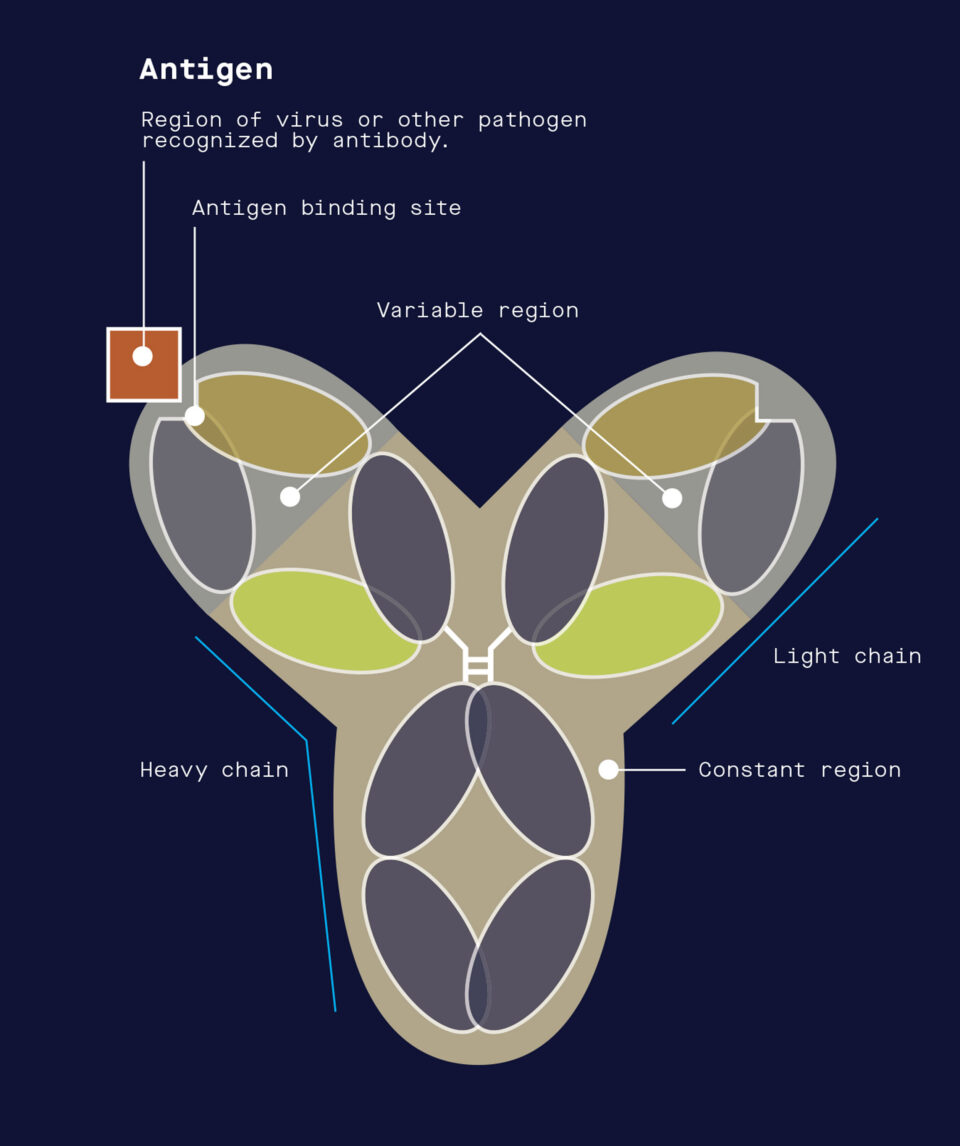
When the immune system encounters a virus or other pathogen, it produces antibodies that neutralize the pathogen by targeting molecular structures referred to as antigens.
In the case of flu, the virus has months to evolve between the time annual vaccine production begins and flu season hits. Adding to the challenge, new strains and subtypes of flu not targeted by the vaccine can enter the human population from animals, mainly pigs and birds. In comparison to flu, many more different strains of HIV circulate among infected people at any given time, and even a single person can carry hundreds of thousands of variants of the virus. A drug or vaccine targeting one strain might work for a time only to have a mutated form emerge that isn’t recognized by the therapies.
The excitement over bnAbs stems from their potential to bring this molecular arms race to an end. The first bnAbs to HIV were discovered in the early nineties by Burton and Carlos Barbas at Scripps Research and by scientists in Vienna, Austria. In 2002, Burton led an effort by the newly formed Neutralizing Antibody Center of IAVI at Scripps Research to find and elicit more bnAbs, preferably with greater potency.
In 2006, after analyzing 1,800 blood samples from HIV-infected people in parts of Africa, India, Southeast Asia, Australia, the United Kingdom and the United States in an effort dubbed IAVI Protocol G, the team found two potent bnAbs in the white blood cells of one woman. In 2009, Burton and his team reported in Science that the woman’s antibodies in laboratory tests proved capable of neutralizing 70 percent of 162 different HIV strains.
In the time since Burton and his colleagues discovered bnAbs against HIV, many others have been identified. Researchers also have deciphered quite a bit about what makes this type of antibody so effective against viruses. Over years of battling HIV in an infected person’s body, the antibodies collect genetic mutations that morph the molecular makeup at the tips of their Y-shaped arms. These adaptions target regions of HIV’s envelope that retain a consistent shape even as other parts of the virus morph, so the antibodies can recognize a broad range of HIV strains. BnAbs are exquisitely adapted to home in on and aggressively bind to HIV. Burton and Wilson discovered, for example, that the antibodies have extra-long loops that act like super Velcro to better cling to HIV virus, which are notoriously devoid of desirable molecular surface features.
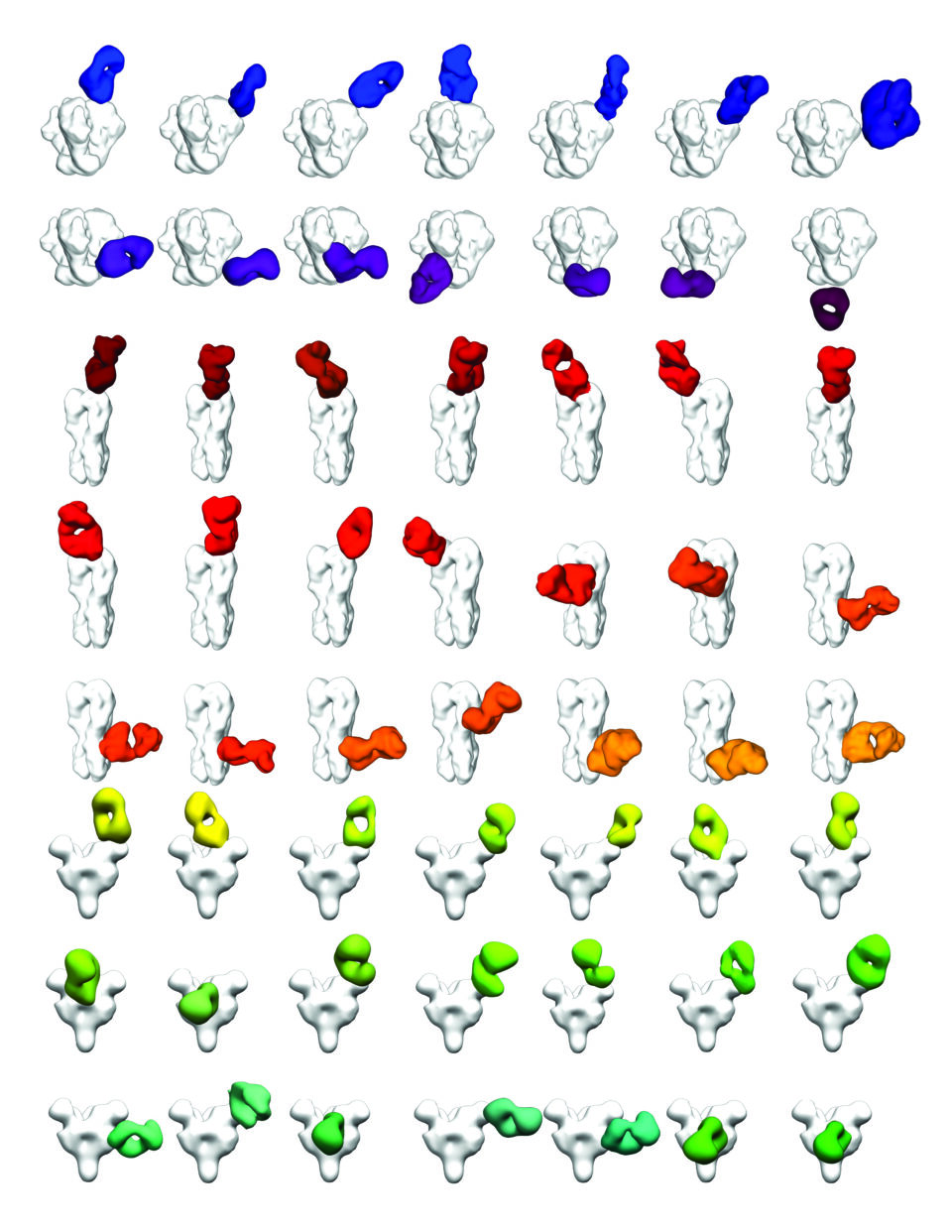
From discovery to design
Merging research on the immune system, viral pathogens, structural biology and vaccine design, the Scripps Research team has recently made significant progress in engineering a series of vaccines that could be administered in stages to coax the immune system to make bnAbs against HIV. Their strategy hinges on a forensic genomic analysis, tracing evolutionary steps that take place in a person’s body between an early progenitor antibody and a fully fledged bnAb. With a roadmap of that progression in hand, they then set out to devise ways to reproduce the process through vaccination.
The state-of-the-art cryo-electron microscopy (cryo-EM) suite at Scripps Research has proven vital to studying the molecular intricacies of how the immune system and viruses interact. Using cryo-EM—which involves deep freezing antibodies and surface antigens from pathogens in liquid ethane and then scanning the samples with an electron beam—the team has captured atomic resolution images of bnAbs binding to HIV and influenza viruses. These efforts, led by Scripps Research Professor Andrew Ward, PhD, have opened new avenues for structural biology research on these difficult targets.
Ward, and his colleague, Scripps Research Professor Lars Hangartner, PhD, recently developed a technique for using cryo-EM to rapidly analyze the outcome of experimental vaccines against HIV and other pathogens. Their new methodology lets scientists quickly assess the full spectrum of antibodies a person produces in response to an infection or vaccine and determine if these antibodies are likely to be effective against the pathogen.
“In the past, much of the interaction between vaccines, the immune system and pathogens was invisible to us,” says Ward, a professor in the Department of Integrative Structural and Computational Biology. “Now we can observe these interactions in detail—at the atomic level, in some cases. It takes much of the guesswork out of determining what’s working and what is not.”
There’s a lot that seems to be working.
Last fall, IAVI announced the start of a phase 1 clinical trial to test a vaccine developed in the laboratory of William Schief, PhD, a professor in the Department of Immunology and Microbiology at Scripps Research. The vaccine is the first in a sequence of engineered vaccine candidates designed to stimulate the immune system to initiate a key first step in the generation of bnAbs against HIV.
“This is just the first step in what would be a multistage vaccination strategy. No one has ever created or even conceived of a vaccine quite like this,” says Schief, who is director of vaccine design for IAVI’s Neutralizing Antibody Center. “If we can make this work for HIV, it could be a model for universal vaccines against other pathogens.”
The other IAVI-led phase 1 HIV-vaccine clinical trial, which was announced this March, will test a different vaccine candidate that Ward and Wilson helped design with John Moore and Rogier Sanders at Weill Cornell Medical College. The vaccine is the first to use a version of the highly fragile outer shell of HIV—a protein called Env that is shaped like a three-pronged spike—that maintains its natural shape. It has shown promising results in preclinical tests.
Flu Snapshot
During the 2016/2017 flu season*, vaccines prevented:
5.3 million
influenza illnesses
2.6 million
medical visits
85,000
hospitalizations
The Scripps Research team is developing “universal” approaches to flu therapy that, if they prove effective in humans, could help prevent many more cases.
*Estimates by Centers for Disease Control and Prevention
“Llamaceuticals” to the rescue
The Scripps Research team is also applying their expertise in bnAb vaccines to develop new therapies for flu and malaria, studies supported by the Bill & Melinda Gates Foundation.
As much as 20 percent of the U.S. population gets the flu each year, and
of those people, around 200,000 are hospitalized. The flu season of 2017-2018 was the worst since 2010, with nearly a million people hospitalized and an estimated 79,000 deaths.
Annual vaccinations help stop the spread of the virus, but the shots inoculate against only a handful of flu strains. Because influenza mutates so quickly, epidemiologists must race each year to predict the identity of the next flu season’s major strains and then crank out a new vaccine accordingly. Even then, the best matched vaccines are only about
40 to 60 percent effective. For certain types and strains of influenza A viruses, vaccine efficacy can drop to as low as 10 to 20 percent. The Scripps Research team made news recently for advances in helping develop an experimental influenza therapy—with the help of llamas.
Using a rare type of antibody produced by the South American cousins of the camel, the Scripps Research scientists and an international team of collaborators, primarily at Janssen, engineered a prototype antibody drug capable of protecting against numerous strains of influenza virus.
“The llama antibodies could be easily linked together to create multi-specific antibodies binding to different sites on different targets. In this case, the multi-specificity was key to having broad coverage of highly variable pathogens like influenza.”
—Ian Wilson, DPhil, Chair of the Department of Integrative Structural and Computational Biology at Scripps Research

The advance, reported in the journal Science last November, received wide attention from media outlets, including The New York Times, Smithsonian Magazine, Axios, BBC and PBS. It even garnered an enthusiastic blog post from Francis Collins, MD, PhD, director of the National Institutes of Health.
To create the drug, the scientists pursued a different approach than for HIV. Instead of prompting the immune system to produce a single broadly neutralizing antibody, they immunized llamas and then engineered a multidomain antibody by tethering together four different llama antibodies, two against influenza A virus and two against influenza B virus. Wilson and Ward led the X-ray and electron microscopy structural studies
to show exactly where this four-in-one antibody was binding to influenza proteins.
“In this case, the llama antibodies could be easily linked together to create multi-specific antibodies binding to different sites on different targets,” Wilson says. “The multi-specificity was key to having broad coverage of highly variable pathogens like influenza.”
Their research showed that when administered as an injection, the antibody could target vulnerable sites on influenza A and B and protect mice against lethal infections. When they tested the multi-domain antibody, it prevented 59 strains of human and avian influenza A and B viruses from multiplying in the mice—an important first step in determining whether the influenza inhibitor could possibly work in people.
Working with scientists at the University of Pennsylvania, the team developed a different delivery mechanism, a “gene mist” containing viral vectors (harmless viruses used to deliver molecular payloads into the genome). When the mist was sprayed into the noses of mice, the vectors carried a genetic blueprint for the engineered antibody into cells of the animals’ respiratory systems. Those cells, now containing the flu-fighting gene in their DNA, produced the antibody, confirming immunity to dangerous strains of flu.
If a similar strategy works in humans, an annual inoculation might still be required, but it would theoretically protect people against far more strains than the seasonal flu vaccine. “And there are other intriguing possible advantages,” Collins wrote of the new strategy. “For example, the rapid protection this approach might afford, along with its potential to neutralize many forms of avian influenza, suggest it might be called into action to help quell an emerging flu pandemic far more swiftly than is possible with traditional vaccines.”
Until then, the team at Scripps Research is on the case.