The FDA in June accepted a New Drug Application for ozanimod, a potential treatment for multiple sclerosis that was created at Scripps Research.
Pharmaceutical company Celgene, which controls rights to the drug and filed the application, said the FDA is expected to issue its final decision on whether to approve ozanimod for relapsing forms of multiple sclerosis—the most common forms of the disease—by March 25 of next year.
“This is a major step in bringing ozanimod to patients with relapsing multiple sclerosis, providing a new treatment option that has shown great promise in clinical testing,” says Hugh Rosen, MD, PhD, who invented ozanimod along with fellow Scripps Research professor Edward Roberts, PhD.
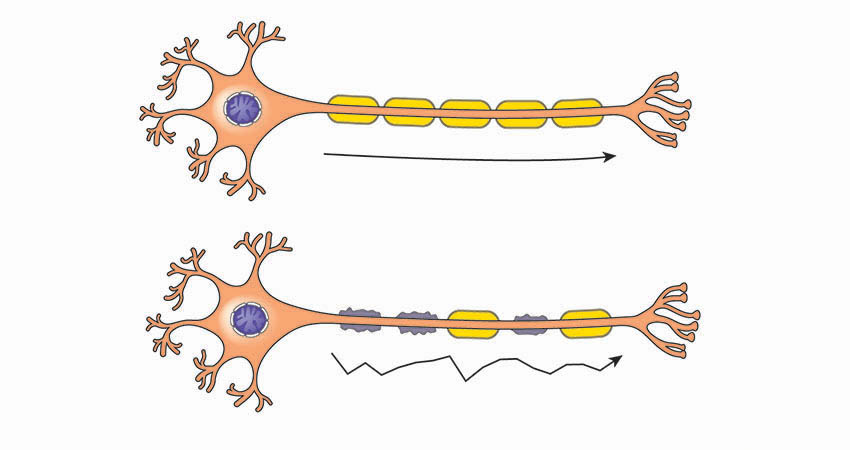
Ozanimod acts on immune cells involved in inflammatory attacks on nerve fibers and the myelin sheaths (pictured) that protect them. Top: healthy; Bottom: diseased.
The European Medicines Agency also accepted an application to market ozanimod in the European Union to adults with relapsing-remitting MS. The applications are based on results from two pivotal phase 3 trials. Treating inflammation is key to reducing disease “flare-ups” among those with relapsing-remitting MS. In the clinical trials, ozanimod significantly reduced brain lesions and brain volume loss compared with the first-line treatment.
In 2015, Celgene acquired the rights to ozanimod, which is also being studied for treating inflammatory bowel disease. Celgene’s phase 3 studies for ulcerative colitis, a form of the disease, are on track to be completed by mid-2020.